 From the May/June 2022 issue. Subscribe today to get your copy
From the May/June 2022 issue. Subscribe today to get your copy
by Erika Villedieu, DVM; William Robinson, BvetMed; Chris Shales, MA, VetMB
From the Department of Soft Tissue Surgery, Willows Veterinary Centre and Referral Service, Solihull, United Kingdom.
Correspondence: [email protected] (E.V.)
Accepted for publication: March 8, 2021.
BOAS (brachycephalic obstructive airway syndrome); OSA (obstructive sleep apnea)
ABSTRACT
Obstructive sleep apnea (OSA) has been uncommonly reported in dogs and is often associated with brachycephalic obstructive airway syndrome (BOAS). OSA independent from BOAS has been rarely reported. Treatment of OSA with ondansetron has only been reported in one dog and has not been reported in a breed commonly affected by BOAS. Here, we report the case of a pug with episodes of OSA despite appropriate treatment of BOAS. Administration of ondansetron led to a rapid and near-complete resolution of the clinical signs, with a follow-up of 3 mo. OSA independent of BOAS should be considered as a differential diagnosis in dogs that present for sleep-disordered breathing without exercise intolerance after appropriate treatment for BOAS. Use of certain serotonin antagonists may be useful as a treatment option for these cases.
Introduction
Sleep disorders are more commonly reported in people, with obstructive sleep apnea (OSA) being one entity.1 OSA has been reported in the English bulldog, mainly as a model for the human disorder.1–3 Relaxation of pharyngeal muscles during sleep has been implicated as a mechanism for OSA in people and in the English bulldog.1,4,5
In the English bulldog and in rats, certain serotonin receptors have been shown to be involved in the occurrence of sleep apnea, and some serotonin antagonists have been studied as potential therapeutic agents for OSA.6–9 Among these, ondansetron has been shown to have some beneficial effects in English bulldogs.3 At present, there is only one report of the use of ondansetron to treat suspected OSA in the clinical setting, in a Chihuahua.10 We report the case of a pug with a suspected diagnosis of OSA despite appropriate treatment of brachycephalic obstructive airway syndrome (BOAS). Administration of ondansetron led to a rapid and near-complete resolution in the clinical signs, despite the presence of laryngeal collapse on laryngoscopy.
Case Report
A 6 kg, 6 yr 6 mo old spayed female pug was presented to our referral hospital for further investigation of episodes of abnormal breathing occurring at rest. The episodes started 2 mo before referral and were described as arousal from sleep, open-mouth breathing, and gasping when sleeping. A high-pitched wheezing noise was also reported accompanying the episodes (Supplementary Video I). The owner specifically described the episodes as occurring almost every time that the dog would go to sleep, and elevation of the head before sleeping would reduce the frequency of the events. No breathing difficulties were reported during waking hours, and the dog was able to exercise in excess of 2.5 hr daily without any evidence of exercise intolerance.
The dog was treated for presumed BOAS by the primary veterinarian, and a staphylectomy and bilateral tonsillectomy were performed 5 wk before presentation to our referral center. The owner reported that the abnormal breathing episodes did not improve after the procedure and that they may have increased in frequency. Treatment with oral prednisolonea 2.5 mg q 24 hr per os and omeprazoleb 5 mg q 24 hr per os was attempted for 2 wk before referral but did not lead to any clinical improvement.
On physical examination, the dog was bright with a normal respiratory rate, respiratory effort, and thoracic auscultation at rest. Intermittent nasal “sniffling” was present. The body condition score was subjectively assessed as 5/9. Functional grading for BOAS, based on respiratory signs and as previously described,11,12 was assessed as grade I at rest (Supplementary Video II). Functional grading was repeated after a 3 min exercise test12 and remained grade I, as evidenced by the absence of upper respiratory noise and the absence of increased respiratory effort (Supplementary Video III). Open-mouth breathing was not noted during exercise. The dog was anesthetized using acepromazinec 0.01 mg/kg IV as a premedication and propofold to effect to achieve a light plane of anesthesia, and a laryngeal examination was performed by a small animal surgery resident and by an ECVS Diplomate in Small Animal Surgery. Grade II to III laryngeal collapse was documented with medial displacement of the corniculate and cuneiform processes and eversion of the laryngeal saccules (Figure 1). The staphylectomy appeared to have been performed at the level of the caudal aspect of the tonsillar crypts.
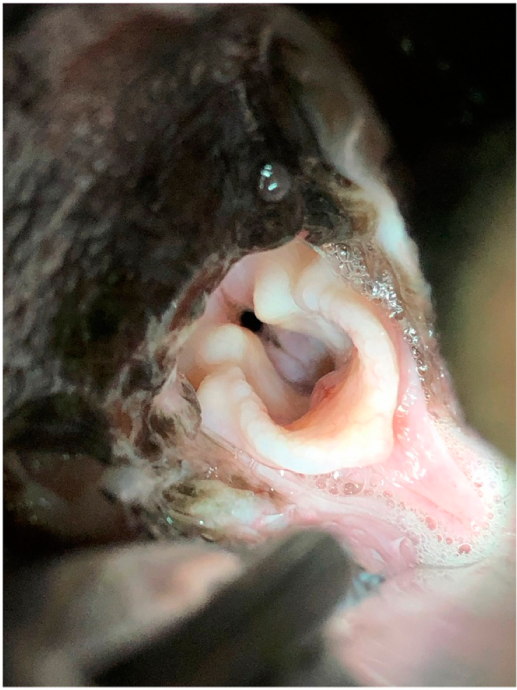 Figure 1: Photograph demonstrating grade II–III laryngeal collapse as assessed during laryngeal examination under general anesthesia.
Figure 1: Photograph demonstrating grade II–III laryngeal collapse as assessed during laryngeal examination under general anesthesia.
Given the discrepancy between the laryngeal examination findings and the level of exercise tolerance, combined with the timing of the breathing difficulty episodes, OSA was suspected to be the cause of the clinical signs. Attempted medical management of OSA was therefore recommended before considering surgical management of the laryngeal collapse. Ondansetrone was prescribed at 4 mg (0.66 mg/kg) q 12 hr for an initial period of 10 days. The owner reported that the OSA events resolved within 24 hr of starting the ondansetron and that the dog appeared to be brighter during the day and sleeping more soundly. The course of ondansetron was continued at the same dose for an additional 3 mo, during which the owner only reported two events of OSA. Re-examination 3 mo after initiation of the ondansetron therapy revealed an unchanged physical examination findings and BOAS functional grading. Treatment with ondansetron at the above-mentioned dose was therefore continued long term. The owner reported that the dog was still doing very well on ondansetron therapy 4.5 mo after the initial presentation.
Discussion
We described the case of a pug with clinical signs consistent with OSA, despite previous surgical treatment of BOAS. Treatment with ondansetron led to resolution of the clinical signs.
Although the dog in this report underwent corrective BOAS surgery before referral, this only included a bilateral tonsillectomy and staphylectomy, and the dog did not undergo a rhinoplasty. Moderately stenotic nares were noted during examination, and the authors would normally perform bilateral alar fold resection and a modified Trader’s rhinoplasty as part of the initial BOAS corrective surgery. Along with stenotic nares, aberrant nasal turbinates have been reported in a majority of dogs affected by BOAS as a contributor to nasal obstruction.13
Diagnosis of aberrant nasal turbinates is usually made using computed tomography examination and rhinoscopy.13,14 In cases in which the aberrant turbinates are considered clinically significant, treatment may be performed using laser-assisted turbinectomy.14 Despite the absence of rhinoplasty in this case, only intermittent sniffling was noted at rest, and nasal breathing persisted during exercise. Although the possibility of nasal obstruction was discussed with the owners, further investigations were not performed in this case, because nasal obstruction was not considered to be a major contributor to the clinical signs. In this case, the improvement with ondansetron medication was sufficient to make the owner unwilling to return for additional surgery to the nares or assessment of the potential presence of clinically relevant aberrant turbinates.
OSA has been described in dogs affected by BOAS, with clinical signs such as difficulties sleeping, arousal from sleep, or apnea during sleep.1,15 However, in these cases, other clinical signs of BOAS are usually present, and the OSA should improve or resolve after appropriate treatment of BOAS. In this report, the dog did not present with any exercise intolerance, stertor, stridor, or increased respiratory effort, and a BOAS functional grade I was attributed. Furthermore, staphylectomy and bilateral tonsillectomy did not result in any improvement in the OSA episodes. Although the dog had evidence of laryngeal collapse on laryngeal examination, there were no clinical signs consistent with severe laryngeal collapse when the dog was conscious, and surgical treatment of the laryngeal collapse was therefore not immediately recommended. It is possible that the level of laryngeal collapse observed during laryngeal examination may have been exacerbated by general anesthesia and relaxation of the laryngeal and pharyngeal muscles. The absence of clear impact of the laryngeal collapse in this case is further supported by the response to therapy without surgical intervention.
Sleep disorders in people are classified as insomnia, sleep-related breathing disorders, hypersomnias of central origin, circadian rhythm sleep disorders, parasomnias, sleep-related movement disorders, isolated symptoms, normal variants, and other sleep disorders.16 Sleep-related breathing disorders are characterized by abnormal respiration during sleep and are classified into central sleep apnea syndromes, OSA syndromes, mixed sleep apnea syndromes, and sleep-related hypoventilation/hypoxemia syndromes, which may be due to underlying medical conditions.16,17
OSA should also be differentiated from dynamic pharyngeal collapse, which occurs in conscious dogs and is often accompanied by other airway abnormalities such as tracheal or bronchial collapse.18 Coughing is the most common clinical sign in dogs with dynamic pharyngeal collapse and was not present in the dog in this report.
OSA has been linked to relaxation of the pharyngeal dilator muscles in people and in dogs, which seems more pronounced during rapid eye movement sleep.1,4,5 In English bulldogs, episodes of sleep apnea were noted more often during rapid eye movement sleep.1 Furthermore, serotonin receptors have been shown to play an important role in the regulation of pharyngeal muscle tone; stimulation of serotonin receptor subtype 5-HT2 leads to increased upper airway dilator muscle tone in the waking state, whereas peripheral stimulation of serotonin receptor subtype 5-HT3 has an inhibitory effect of respiration.6,9,19 The use of serotonin agonists and antagonists has therefore been studied as a possible management option for OSA. Ondansetron and mirtazapine, both 5-HT3 receptor antagonists, were shown to reduce the incidence of sleep apnea in rats.7,8 Trazodone combined with L-tryptophan also reduced the frequency of sleep apnea events in English bulldogs.2
Ondansetron is a serotonin antagonist acting on the 5-HT3 receptor subtype, and it most likely acts by counteracting pharyngeal muscle relaxation mediated by stimulation of 5-HT3 receptors in the nodose ganglion.3,19 Ondansetron has been used in English bulldogs with OSA and led to a reduction in the sleep apnea events during rapid eye movement sleep.3 However, in that study, ondansetron was administered as a single dose and at a higher dose (2 mg/kg) than normally recommended for clinical use. In a previous case report, ondansetron was successfully used to manage OSA symptoms in a Chihuahua.10 In people, the most common management option for OSA is continuous positive airway pressure, but the use of ondansetron combined with fluoxetine led to a decrease in the apnea hypopnea index in one study.19 In the case reported here, the owners were counselled that the use of ondansetron was based on a presumptive diagnosis of OSA and that this was an empirical therapy with little evidence-based support.
One limitation of this report is the difficulty in objectively diagnosing OSA as a cause for the reported episodes. In a patient affected by BOAS, the lack of improvement after initial surgery should prompt investigations into more common causes of continued airway obstruction, such as aberrant nasal turbinates, nasopharyngeal obstruction, macroglossia, laryngeal obstruction, or tracheal disorders. Whole-body barometric plethysmography has been used to diagnose sleep-disordered breathing in Cavalier King Charles spaniels,15 but this is not a widely available modality. Dynamic pharyngeal collapse has been diagnosed using fluoroscopy or four-dimensional computed tomography during spontaneous respiration in dogs.20,21 However, the use of conscious fluoroscopy in the dog in this report would likely not have been beneficial in diagnosing OSA, and the use of fluoroscopy during sleep is impractical. This report highlights the potential benefit of considering sleep disorders and treatment of such alongside assessment and appropriate multilevel surgery for animals also suffering from significant BOAS. The authors do not consider that ondansetron treatment should replace anatomical BOAS assessment and treatment.
Conclusion
We report a case of suspected OSA in a pug previously treated for BOAS. Long-term treatment with ondansetron led to resolution of the OSA episodes. Although OSA in a brachycephalic breed and in the absence of clinical signs of BOAS appears to be an uncommon presentation, this diagnosis should be considered in dogs who have been appropriately treated for BOAS and are showing no exercise intolerance. Ideally, OSA should be a diagnosis of exclusion after ruling out other causes of persistent airway obstruction. Use of serotonin antagonist may prove to be a useful therapeutic option in these patients.
Footnotes
aPrednisolone tablets B.P.(Vet) 5 mg; Millpedge Veterinary, Retford, United Kingdom
bOmeprazole 10 mg gastro-resistant capsules; Milpharm, South Ruislip, United Kingdom
cAcecare 2 mg/mL; Animalcare, York, United Kingdom
dPropofol-Lipuro Emulsion for Injection; Virbac, Bury St. Edmunds, United Kingdom
eOndansetron 8 mg film-coated tablets; Pliva, Zagreb, Croatia
Supplementary data (videos)
REFERENCES
1. Hendricks JC, Kline LR, Kovalski RJ, et al. The English bulldog: a natural model of sleep-disordered breathing. J Appl Physiol 1987; 63( 4): 1344– 50.
2. Veasey SC, Fenik P, Panckeri K, et al. The effects of trazodone with L-tryptophan on sleep-disordered breathing in the English bulldog. Am J Respir Crit Care Med 1999; 160: 1659– 67.
3. Veasey SC, Chachkes J, Fenik P, et al. The effects of ondansetron on sleep-disordered breathing in the English bulldog. Sleep 2001; 24( 2): 155– 60.
4. Okabe S, Hida W, Kikuchi Y, et al. Upper airway muscle activity during REM and non-REM sleep of patients with obstructive apnea. Chest 1994; 106( 3): 767– 73.
5. Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med 1996; 153( 6): 1880– 7.
6. Veasey SC, Veasey SC, Panckeri KA, et al. The effects of serotonin antagonists in an animal model of sleep-disordered. Am J Respir Crit Care Med 1996; 153( 2): 776– 86.
7. Radulovacki M, Trbovic SM, Carley DW. Serotonin 5-HT3-receptor antagonist GR 38032F suppresses leep apneas in rats. Sleep 1998; 21( 2): 131– 6.
8. Carley DW Radulovacki M. Mirtazapine, a mixed-profile serotonin agonist/antagonist, suppresses sleep apnea in the rat. Am J Respir Crit Care Med 1999; 160( 12): 1824– 9.
9. Veasey SC. Serotonin agonists and antagonists in obstructive sleep apnea. Am J Respir Med 2003; 2: 21– 9.
10. Kopke MA, Wightman P, Ruaux CG. Obstructive sleep apnea in a Chihuahua successfully managed with ondansetron. Clin Case Rep 2019; 7: 872– 6.
11. Poncet CM, Dupre GP, Freiche VG, et al. Long-term results of upper respiratory syndrome surgery and gastrointestinal tract medical treatment in 51 brachycephalic dogs. J Small Anim Pract 2006; 47: 137– 42.
12. Riggs J, Liu N, Sutton DR, et al. Validation of exercise testing and laryngeal auscultation for grading brachycephalic obstructive airway syndrome in pugs, French bulldogs, and English bulldogs by using whole-body barometric plethysmography. Vet Surg 2019; 48: 488– 96.
13. Oechtering GU, Pohl S, Schlueter C, et al. A novel approach to brachycephalic syndrome. 1. Evaluation of anatomical intranasal airway obstruction. Vet Surg 2016; 45( 2): 165– 72.
14. Oechtering GU, Pohl S, Schlueter C, et al. A novel approach to brachycephalic syndrome. 2. Laser-assisted turbinectomy (LATE). Vet Surg 2016; 45( 2): 173– 81.
15. Hinchliffe TA, Liu N, Ladlow J. Sleep-disordered breathing in the Cavalier King Charles spaniel: a case series. Vet Radiol 2019; 48: 497– 504.
16. American Academy of Sleep Medicine. The international classification of sleep disorders–diagnostic and coding manual. 2nd ed. Westchester (IL): American Academy of Sleep Medicine; 2005.
17. Khan M Franco R. Complex sleep apnea syndrome. Sleep Disord 2014; 2014: 798487.
18. Rubin JA, Holt DE, Reetz JA, et al. Signalment, clinical presentation, concurrent diseases, and diagnostic findings in 28 dogs with dynamic pharyngeal collapse (2008–2013). J Vet Intern Med 2015; 29: 815– 21.
19. Prasad B, Radulovacki M, Olopade C, et al. Prospective trial of efficacy and safety of ondansetron and fluoxetine in patients with obstructive sleep apnea syndrome. Sleep 2010; 33( 7): 982– 9.
20. Pollard RE, Johnson LR, Marks SL. The prevalence of dynamic pharyngeal collapse is high in brachycephalic dogs undergoing videofluoroscopy. Vet Radiol Ultrasound 2018; 59: 529– 34.
21. Hara Y, Teshima K, Seki M, et al. Pharyngeal contraction secondary to its collapse in dogs with brachycephalic airway syndrome. J Vet Med Sci 2020; 82( 1): 64– 7. Google



